Enovita®
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionPeer-reviewed science on Enovita®
The most recent study on Enovita® included 80 healthy participants that were randomized to receive GSE supplementation or placebo twice a day for a total of 16 weeks. The principal endpoints included pressure modulation and the perception of stress and worries.1
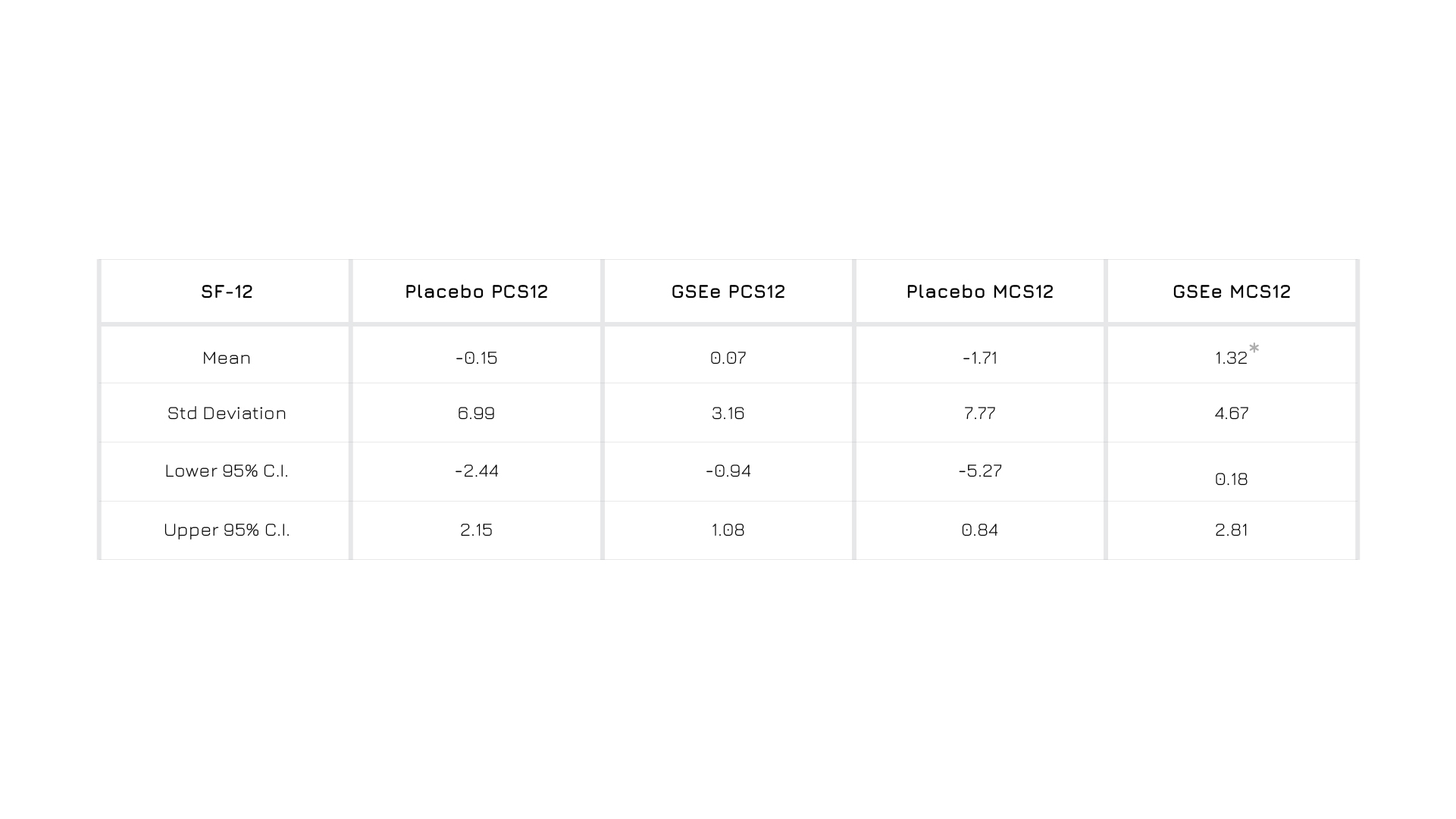
Enovita® supplementation is associated with a general increase of mental quality of life (quality of life questionnaire SF-12 and mental component summary MCS12).1
Figure 1. Results from SF-12 self-administered questionnaire.
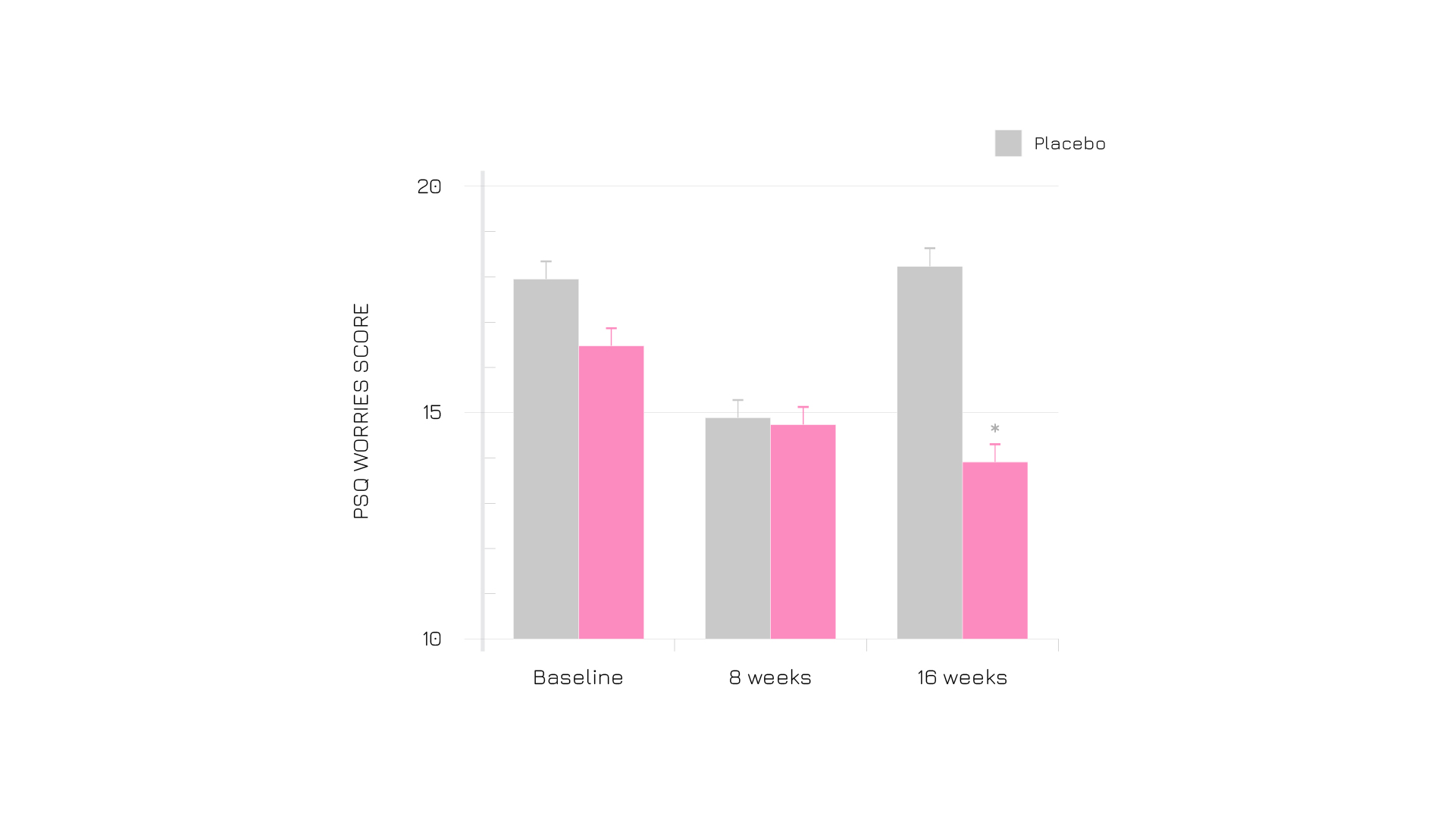
Also the perception of worries under Enovita® supplementation was less pronounced (Figure 2).
Figure 2. Results from PSQ self-administered questionnaire at baseline, after 8 and 16 weeks. (Validated tool to assess subjectively experienced stress independent of a specific or objective occasion). Graphic elaboration of table 2, form ref. 1.
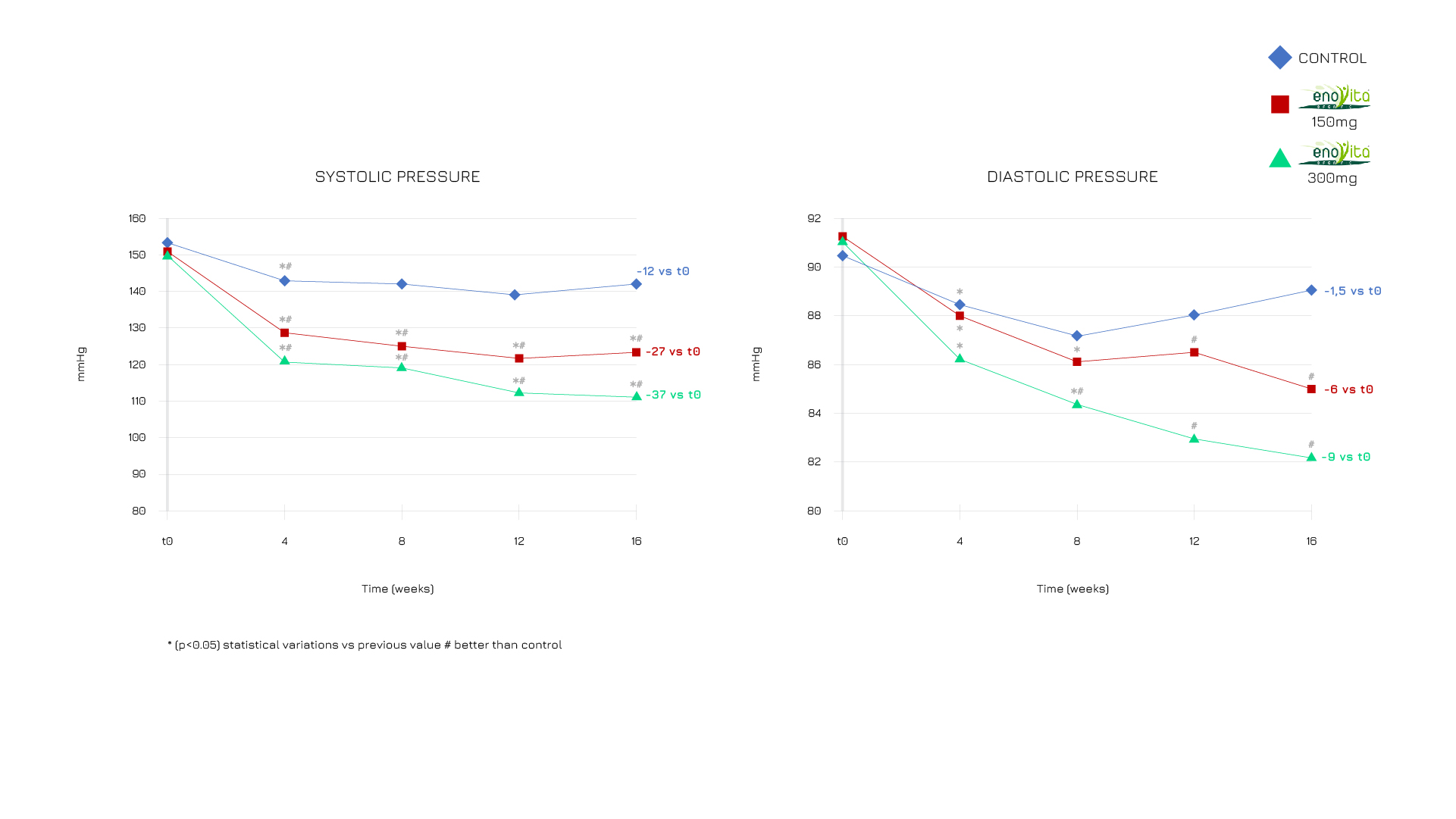
Enovita® Organic significantly modulated both systolic and diastolic pressurewith a dose-dependent effect.1
Figure 3. A favorable effect of systolic blood pressure was observed in all groups, but the effect was significantly higher in the treatment group (p < 0.05). The effect was lower for the diastolic pressure. Thus, in the 2 treatment groups, an effect on the diastolic pressure was also observed and developed more gradually in time.1
Enovita® can have beneficial cardiovascular effects that may support intervention strategies in cardiac stress.
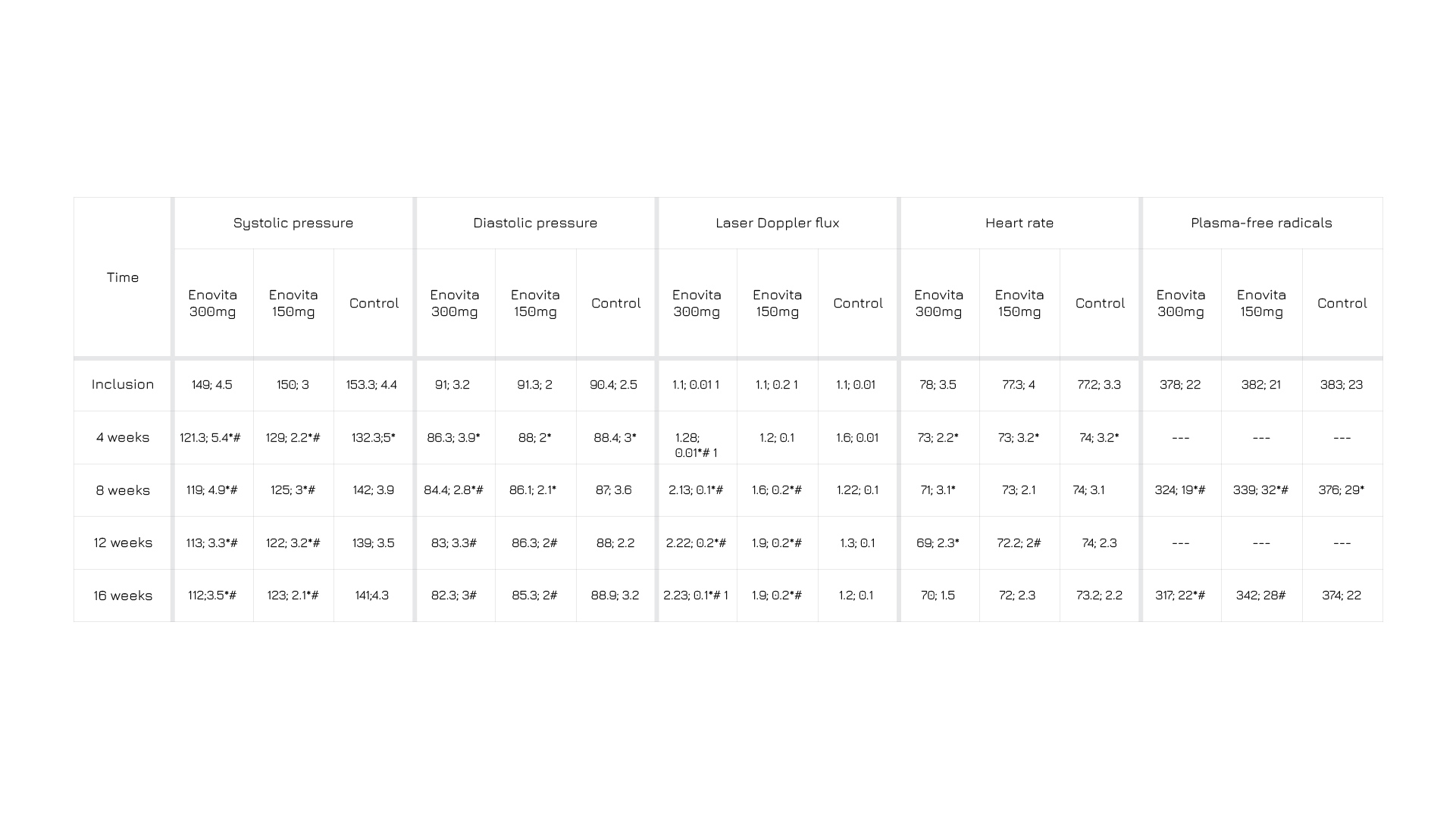
In a human study, after 4 months of administration, a statistically significant, and dose-dependent, amelioration in all endpoints (systolic pressure, diastolic pressure, laser doppler flux, heart rate, plasma-free radicals) is observed in both groups delivered with Enovita® compared to the not delivered one, with blood pressure normalizing in 93% of the higher dosage (300mg) administrated group. Taken together, these observations suggest that Enovita® have beneficial cardiovascular effects that may support current management strategies in the hypertension area.
[TABLE TITLE] Systolic pressure, diastolic pressure, laser doppler flux, heart rate, plasma-free radicals in 2 Enovita® administrated groups (300 mg and 150 mg) and a not administrated one, at inclusion, 4, 8, 12, and 16 weeks.
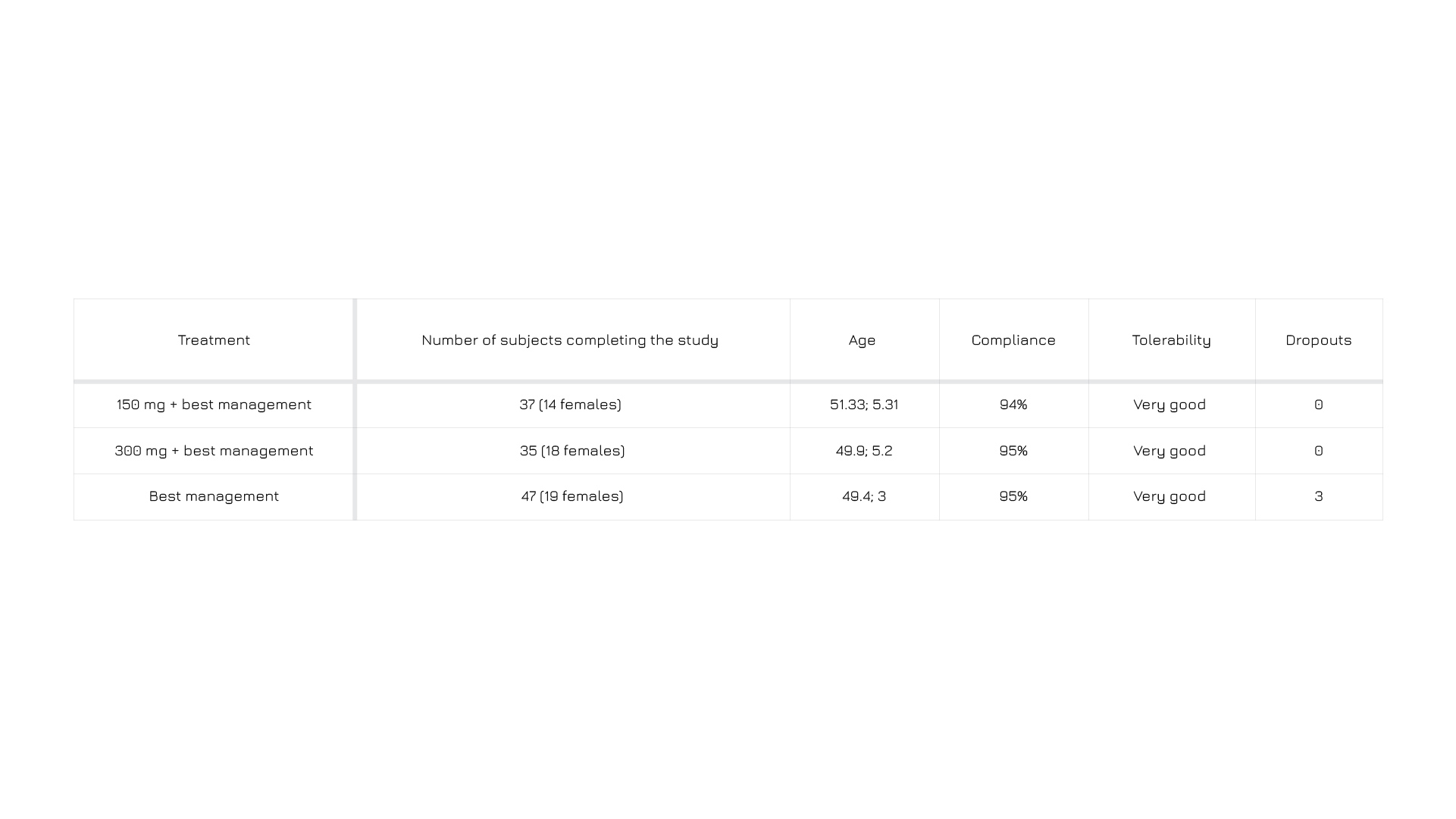
[TABLE TITLE] Subjects compliance and tolerability at the end of the trial.
BIBLIOGRAPHY
1Schön C, et al. Nutrients. 13(2):654. (2021)
2Belcaro G., Ledda A., Hu S., et al., Evidence-Based Complementary and Alternative Medicine, ID 313142 (2013).
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.